
Global Mucopolysaccharidosis Treatment Market to Witness Robust Growth, Projected to Reach USD 5,014.5 Million by 2035 Growing at a CAGR of 5.9% | FMI
Rising health awareness, increasing prevalence of various forms of mucopolysaccharidosis worldwide, continuous advances in medical research and new drug launches and approvals are some of the factors driving the mucopolysaccharidosis treatment market. Mucopolysaccharidosis is a group of inherited disorders in which the body is unable to breakdown glycosaminoglycan (long chains of sugars) due to absence or malfunctioning of certain enzymes. Presence of these disorders badly affects the physical and mental development of a person as well as damages the organs.
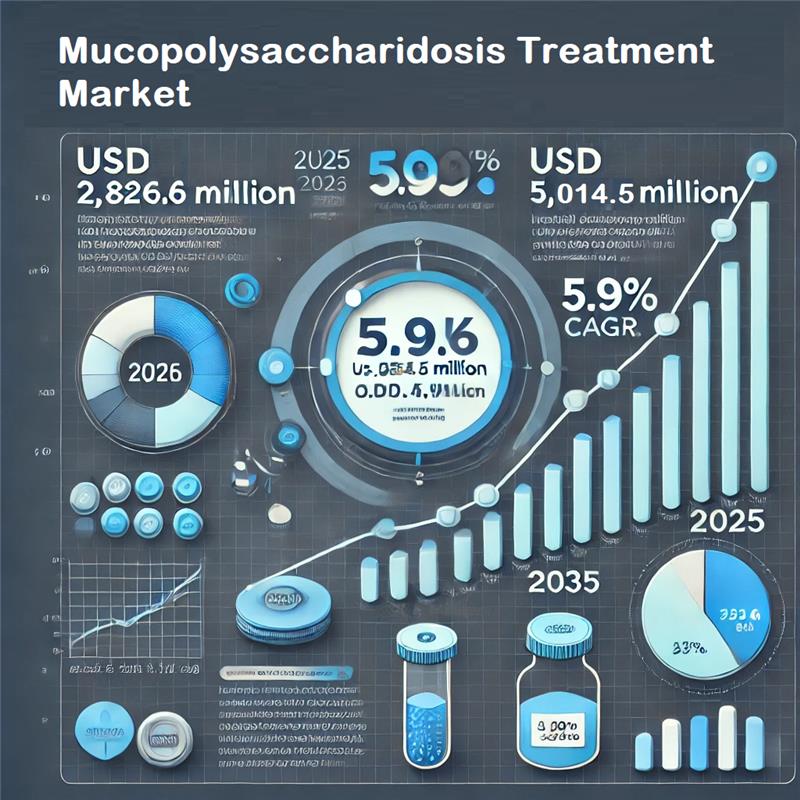
/EIN News/ -- NEWARK, Del, Jan. 02, 2025 (GLOBE NEWSWIRE) -- The global mucopolysaccharidosis treatment market, valued at USD 2,669.1 million in 2024, is estimated to grow at a compound annual growth rate (CAGR) of 5.9% during the forecast period of 2025 to 2035. By the end of 2025, the market is projected to reach USD 2,826.6 million, with sales anticipated to soar to an impressive USD 5,014.5 million by 2035. This represents a year-on-year growth of 5.8% in 2024, underlining the increasing demand for effective treatments for this rare genetic disorder.
MPS, a rare and debilitating genetic condition, occurs when cells fail to produce the specific enzymes needed to break down mucopolysaccharides. This deficiency results in the accumulation of harmful substances within cells, leading to progressive organ damage and developmental challenges. Treatment approaches such as enzyme replacement therapy (ERT) and hematopoietic stem cell transplantation (HSCT) have become cornerstones in managing the disease.
How Will Focus on Clinical Trials Affect Mucopolysaccharidosis Treatment Market?
Clinical trials has remained the key focus of mucopolysaccharidosis treatment during the last few years. Amid rise in the cases of rare diseases, academic researchers, clinicians and leading market players are increasingly focusing on developing new treatment options that can significantly relieve the symptoms and help the patients to lead a normal life.
Increasing clinical research is in turn creating lucrative growth prospects within mucopolysaccharidosis treatment market and the trend is likely to continue in future.
Various regulatory bodies are approving the clinical trials of innovative treatments. For instance, in February 2020 – FDA granted fast track designation to Lysogene's LYS-SAF302 program for the treatment of MPS IIIA. Currently the new second generation gene therapy (LYS-SAF302) is being investigated in the international phase 2/3 clinical trial.
Similarly in Feb 2021, JCR Pharmaceuticals Co., Ltd. received clearance of its Investigational New Drug (IND) application from the US Food and Drug Administration (FDA) to initiate a phase 3 clinical trial of JR-141 for the treatment of MPS II (Hunter syndrome) in the US.
Consistent clinical trials conducted to encourage the discovery of novel therapeutics is anticipated to expand the growth of mucopolysaccharidosis treatment market during the forecast period
What will be the Key Restraints to Mucopolysaccharidosis Treatment Market Growth?
One of the major factors restraining the growth of mucopolysaccharidosis treatment market is the high cost associated with mucopolysaccharidosis treatment. Both ERT and stem cell therapies treatment are expensive. As a result, a large section population are not able to afford advanced treatments.
Moreover, lack of favorable reimbursement policies across and poor health awareness are limiting the growth of the market.
Drivers of Market Growth:
The growth of the MPS treatment market is attributed to:
- Advancements in Biotechnology: Innovations in therapeutic techniques, including gene therapy research, have significantly improved treatment outcomes for MPS patients.
- Enhanced Diagnostic Methods: Improved tools for early diagnosis are enabling timely interventions, enhancing the efficacy of treatments.
- Increased Awareness of Rare Diseases: Educational campaigns and initiatives have elevated public and professional understanding of MPS, leading to greater adoption of treatments.
- Availability of ERT: The widespread accessibility of enzyme replacement therapy has improved patients' quality of life by effectively managing symptoms.
Future Outlook:
The ongoing research into gene therapies and the development of next-generation treatments are expected to revolutionize the MPS treatment landscape. These advancements, coupled with supportive care that targets symptom management, continue to elevate patient outcomes and the overall market potential.
growth, driven by a convergence of technological advancements and increasing awareness of rare genetic disorders. Enhanced diagnostic capabilities not only ensure early detection but also improve patient access to life-altering treatments such as enzyme replacement therapy and emerging gene therapies. The sustained focus on research and development is expected to unlock further innovations, addressing the unmet needs of this underserved patient population,” says Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.).
Category-wise Insights
Which is the Most Preferred Treatment for Mucopolysaccharidosis?
“Enzyme Replacement Therapy (ERT) Remains the Gold Standard Treatment for Mucopolysaccharidosis”
Based on treatment, mucopolysaccharidosis treatment market is segmented into enzyme replacement therapies (ERT) and stem cell therapies. Among these two, enzyme replacement therapies segment dominates the mucopolysaccharidosis treatment market.
ERT remains the primary treatment option for mucopolysaccharidosis across the globe. Easy availability and continuous approvals remain the key factors fueling the adoption of enzymes replacement therapies for relieving the symptoms of mucopolysaccharidosis.
Enzyme replacement therapies replace or generate the deficient enzyme and thus effectively helps to treat the condition. Drugs such as aldurazyme, naglazyme, vimizim, elaprase, mepsevii and hunterase are being consumed on large scales for the treatment of mucopolysaccharides.
Which Type of MSP Dominates the Mucopolysaccharidosis Treatment Market?
“MPS II Segment to Continue Accounting for Maximum Sales”
In terms of MPS type, the mucopolysaccharidosis treatment has been segmented into MPS I, MPS II, MPS IV A, MPS VI and MPS VII. Among these, MPS II (Hunter syndrome) segment dominates the global mucopolysaccharidosis treatment market, accounting for the largest share of 33.2% in 2022.
MPS II or hunter syndrome is a rare inherited genetic disorder caused by absence or malfunctioning of enzyme 2-sulfatas. Absence of this enzyme causes permanent progressive damage affecting mental development, appearance and organ functions. As a result, medical products such as hunterase and elaprase are being consumed to relieve the symptoms.
Increasing prevalence of MPS II coupled with presence of multiple treatment options is triggering the growth of MPS II segment and the trend is likely to continue in the future.
Who is the Leading End User of Mucopolysaccharidosis Treatment?
“Favorable Scenario Enables Dominance of Hospitals Segment”
In terms of end user, the global donor egg IVF market is segmented into hospital, specialty clinics, medical research centers and home-infusion.
Among these, the hospitals segment accounts for major revenue share in the global mucopolysaccharides treatment market in 2024. Presence of trained medical professionals and low-cost treatment are some of the factors prompting patients to opt for mucopolysaccharidosis treatment in hospitals.
Hospitals are expected to remain the only means of accessing the treatment for maximum number of people living in developing and underdeveloped regions. However, specialty clinics and home infusion segments are anticipated to grow at a robust CAGR over the forecast period.
Get Full Report Now: https://www.futuremarketinsights.com/reports/mucopolysaccharidosis-treatment-market
Recent Developments in the Mucopolysaccharidosis Treatment Industry:
- December 2024: MEDIPAL HOLDINGS CORPORATION and JCR Pharmaceuticals Co., Ltd., commenced a Phase I/II clinical trial for JR-446 in Japan. JR-446 is a novel blood-brain barrier (BBB)-penetrating α-N-acetylglucosaminidase under development for treating mucopolysaccharidosis type IIIB.
- April 2023: JCR Pharmaceuticals and Sumitomo Pharma entered into a co-promotion agreement for IZCARGO in Japan. IZCARGO is a recombinant therapeutic specifically designed for mucopolysaccharidosis type II.
Key Players of Mucopolysaccharidosis Treatment Industry:
- Sanofi S.A.
- Shire
- BioMarin Pharmaceutical Inc.
- Ultragenyx Pharmaceutical Inc.
- Sarepta Therapeutics
- Abeona Therapeutics, Inc.
- ArmaGen
- Eloxx Pharmaceuticals
- Esteve
- Immusoft Corporation
- Inventiva
- Orchard Therapeutics Limited
- REGENXBIO Inc.
- Sangamo Therapeutics, Inc.
Key Segments of Mucopolysaccharidosis Treatment Market:
By Treatment:
In terms of treatment, the industry is divided into enzyme replacement therapies and stem cell therapies (Bone marrow transplantation (BMT) and Umbilical cord blood transplantation (UCBT))
By Type of MPS:
The industry is classified by type of MPS as MPS I, MPS II, MPS IV A, MPS VI and MPS VII
By End User:
The industry is classified by Hospital, Specialty Clinics, Medical Research Centers, Home-infusion
By Region:
Key countries of North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia and Pacific, and Middle East and Africa (MEA) have been covered in the report.
French Translate:
Le marché mondial du traitement de la mucopolysaccharidose connaîtra une croissance robuste, qui devrait atteindre 5 014,5 millions USD d’ici 2035, avec un TCAC de 5,9 % | L’IGF
Le marché du traitement de la mucopolysaccharidose (MPS) devrait connaître une expansion significative, stimulée par les progrès de la biotechnologie, l’amélioration des techniques de diagnostic et la sensibilisation croissante aux maladies rares.
Le marché mondial du traitement de la mucopolysaccharidose, évalué à 2 669,1 millions USD en 2024, devrait croître à un taux de croissance annuel composé (TCAC) de 5,9 % au cours de la période de prévision de 2025 à 2035. D’ici la fin de 2025, le marché devrait atteindre 2 826,6 millions USD, avec des ventes qui devraient atteindre un impressionnant 5 014,5 millions USD d’ici 2035. Cela représente une croissance de 5,8 % en glissement annuel en 2024, soulignant la demande croissante de traitements efficaces pour cette maladie génétique rare.
La MPS, une maladie génétique rare et débilitante, se produit lorsque les cellules ne parviennent pas à produire les enzymes spécifiques nécessaires à la décomposition des mucopolysaccharides. Cette carence entraîne l’accumulation de substances nocives dans les cellules, entraînant des lésions organiques progressives et des problèmes de développement. Les approches thérapeutiques telles que l’enzymothérapie substitutive (ERT) et la greffe de cellules souches hématopoïétiques (HSCT) sont devenues des pierres angulaires dans la prise en charge de la maladie.
Moteurs de la croissance du marché :
La croissance du marché du traitement MPS est attribuée à :
1. Progrès en biotechnologie : Les innovations dans les techniques thérapeutiques, y compris la recherche sur la thérapie génique, ont considérablement amélioré les résultats du traitement pour les patients atteints de MPS.
2. Méthodes de diagnostic améliorées : Des outils améliorés pour le diagnostic précoce permettent des interventions opportunes, améliorant ainsi l’efficacité des traitements.
3. Sensibilisation accrue aux maladies rares : Les campagnes et les initiatives éducatives ont permis au public et aux professionnels de mieux comprendre la MPS, ce qui a conduit à une plus grande adoption des traitements.
4. Disponibilité de l’ERT : L’accessibilité généralisée de l’enzymothérapie substitutive a amélioré la qualité de vie des patients en gérant efficacement les symptômes.
Perspectives d’avenir :
La recherche en cours sur les thérapies géniques et le développement de traitements de nouvelle génération devraient révolutionner le paysage du traitement MPS. Ces avancées, associées à des soins de soutien qui ciblent la gestion des symptômes, continuent d’améliorer les résultats pour les patients et le potentiel global du marché.
« Le marché du traitement de la mucopolysaccharidose connaît une croissance remarquable, stimulée par une convergence des avancées technologiques et une sensibilisation croissante aux maladies génétiques rares. Les capacités de diagnostic améliorées garantissent non seulement une détection précoce, mais améliorent également l’accès des patients aux traitements qui changent la vie, tels que l’enzymothérapie substitutive et les thérapies géniques émergentes. L’accent mis sur la recherche et le développement devrait débloquer d’autres innovations, répondant aux besoins non satisfaits de cette population de patients mal desservis », a déclaré Sabyasachi Ghosh (vice-président associé chez Future Market Insights, Inc.).
Paysage concurrentiel :
L’industrie pharmaceutique réalise d’importants investissements dans le traitement de la mucopolysaccharidose (MPS), en mettant l’accent sur le développement de candidats-médicaments innovants prêts à être lancés sur le marché. Cet accent stratégique est à l’origine d’une croissance substantielle sur le marché du traitement MPS. De plus, les efforts accrus de l’industrie pour lancer et commercialiser de nouveaux produits contribuent davantage à cette trajectoire ascendante.
Développements récents dans l’industrie du traitement de la mucopolysaccharidose :
- Décembre 2024 : MEDIPAL HOLDINGS CORPORATION et JCR Pharmaceuticals Co., Ltd. ont commencé un essai clinique de phase I/II pour le JR-446 au Japon. Le JR-446 est une nouvelle α-N-acétylglucosaminidase pénétrant la barrière hémato-encéphalique (BHE) en cours de développement pour le traitement de la mucopolysaccharidose de type IIIB.
- Avril 2023 : JCR Pharmaceuticals et Sumitomo Pharma ont conclu un accord de co-promotion pour IZCARGO au Japon. IZCARGO est un traitement recombinant spécialement conçu pour la mucopolysaccharidose de type II.
Principaux acteurs de l’industrie du traitement de la mucopolysaccharidose :
- Sanofi S.A.
- Comté
- BioMarin Pharmaceutical Inc.
- Ultragenyx Pharmaceutical Inc.
- Sarepta Therapeutics
- Abeona Therapeutics, Inc.
- ArmaGen
- Eloxx Produits pharmaceutiques
- Estève
- Immusoft Corporation
- Inventiva
- Orchard Therapeutics Limited
- REGENXBIO Inc.
- Sangamo Therapeutics, Inc.
Segments clés du marché du traitement de la mucopolysaccharidose :
Par traitement :
En termes de traitement, l’industrie est divisée en thérapies enzymatiques substitutives et thérapies à base de cellules souches (greffe de moelle osseuse (BMT) et transplantation de sang de cordon ombilical (UCBT))
Par type de MPS :
L’industrie est classée par type de MPS comme suit : MPS I, MPS II, MPS IV A, MPS VI et MPS VII
Par utilisateur final :
L’industrie est classée par hôpital, cliniques spécialisées, centres de recherche médicale, perfusion à domicile
Par région :
Les principaux pays d’Amérique du Nord, d’Amérique latine, d’Europe occidentale, d’Europe de l’Est, d’Asie de l’Est, d’Asie du Sud et du Pacifique, ainsi que du Moyen-Orient et de l’Afrique (MEA) ont été couverts par le rapport.
Author By:
Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.) holds over 12 years of experience in the Healthcare, Medical Devices, and Pharmaceutical industries. His curious and analytical nature helped him shape his career as a researcher.
Identifying key challenges faced by clients and devising robust, hypothesis-based solutions to empower them with strategic decision-making capabilities come naturally to him. His primary expertise lies in areas such as Market Entry and Expansion Strategy, Feasibility Studies, Competitive Intelligence, and Strategic Transformation.
Holding a degree in Microbiology, Sabyasachi has authored numerous publications and has been cited in journals, including The Journal of mHealth, ITN Online, and Spinal Surgery News.
Explore FMI’s Related Ongoing Coverage on Healthcare Domain:
The global enzyme replacement therapy market size, valued at an impressive USD 10,707.6 million in 2024, is poised for significant expansion, with a projected Compound Annual Growth Rate (CAGR) of 6.60% from 2024 to 2034. This remarkable growth trajectory is set to propel the market to a valuation of USD 20,289.1 million by the close of 2034.
The global sales of in-ear-monitors (IEMs) are estimated to be worth USD 410.4 million in 2025 and anticipated to reach a value of USD 687.9 million by 2035. Sales are projected to rise at a CAGR of 5.3% over the forecast period between 2025 and 2035.
The global home sleep apnea testing market share are estimated to be worth USD 712.3 million in 2025 and are anticipated to reach a value of USD 966.1 million by 2035. Sales are projected to rise at a CAGR of 3.1% over the forecast period between 2025 and 2035.
The global vacuum mixing devices market growth is estimated to be worth USD 169.5 million in 2025 and anticipated to reach a value of USD 214.9 million by 2035. Sales are projected to rise at a CAGR of 2.4% over the forecast period between 2025 and 2035.
The pancreatic stone protein testing market demand is estimated to reach USD 2.2 billion in 2024. It is anticipated to grow at a CAGR of 2.3% during the assessment period 2024 to 2034 and reach a value of USD 2.8 billion by 2034.
The global cardiac surgery devices market outlook is estimated to be worth USD 1.8 billion in 2024 and is projected to grow at a CAGR of 3.7%, reaching USD 2.5 billion by 2034.
The global lacrimal devices market overview are estimated to be worth USD 32.1 million in 2025 and anticipated to reach a value of USD 57.0 million by 2035. Sales are projected to rise at a CAGR of 5.9% over the forecast period between 2025 and 2035.
The global knotless tissue control devices market strategies are estimated to be worth USD 494.4 million in 2025 and are anticipated to reach a value of USD 728.6 million by 2035. Sales are projected to rise at a CAGR of 2.7% over the forecast period between 2025 and 2035.
The global transcutaneous monitors market development are estimated to be worth USD 455.9 million in 2025 and anticipated to reach a value of USD 694.6 million by 2035. Sales are projected to rise at a CAGR of 4.3% over the forecast period between 2025 and 2035.
The global robotic catheterization systems market opportunity are estimated to be worth USD 54.4 million in 2025 and anticipated to reach a value of USD 190.2 million by 2035. Sales are projected to rise at a CAGR of 13.3% over the forecast period between 2025 and 2035.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube

Distribution channels: Book Publishing Industry ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release


